Masimo kondigt CE-markering voor rainbow® Super-sensor aan
NEUCHATEL, Zwitserland–(BUSINESS WIRE)– Masimo (NASDAQ: MASI) heeft een CE-markering gekregen voor de rainbow ® Super DCI ®-minisensor, een herbruikbare sensor voor verscheidene steekproefsgewijze fysiologische metingen met puls-oximetrie met Masimo’s technologieën Measure-through Motion, Low Perfusion™ en rainbow SET™. Voor het eerst is het mogelijk met dezelfde niet-invasieve sensors hemoglobine, carboxyhemoglobine, methemoglobine en de zuurstofsaturatie in de slagaders te meten.
Masimo Announces CE Marking of rainbow® Super Sensor |
|||||
|
NEUCHATEL, Switzerland–(BUSINESS WIRE)– Masimo (NASDAQ: MASI) announced today the CE marking of the rainbow ® Super DCI ®-mini sensor, a reusable spot-check sensor that features Masimo SET ® Measure-through Motion and Low Perfusion™ pulse oximetry and rainbow SET™ technology with multiple physiologic measurements – including, for the first time, the ability to measure total hemoblogin (SpHb ®), carboxyhemoglobin (SpCO ®), methemoglobin (SpMet ®), and arterial oxygen saturation (SpO 2) using the same noninvasive reusable sensor. This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170709005051/en/ 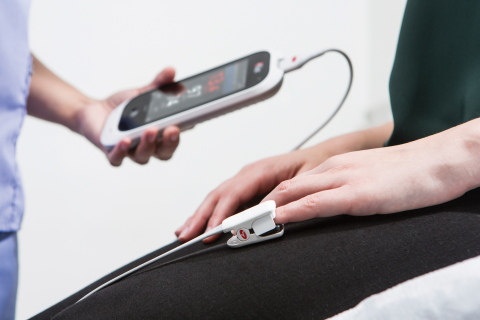 Masimo Rad-67™ Pulse CO-Oximeter® with rainbow® Super DCI®-mini Sensor (Photo: Business Wire) Masimo Rad-67™ Pulse CO-Oximeter® with rainbow® Super DCI®-mini Sensor (Photo: Business Wire)
In 2016, Masimo introduced the rainbow ® DCI-mini sensor, enabling spot-check measurement of Next Generation SpHb and other parameters. Now, with the rainbow ® Super DCI-mini sensor, an expanded set of parameters can be measured using a single sensor: SpO 2, pulse rate (PR), perfusion index (Pi), pleth variability index (PVi ®), SpHb, SpCO, and SpMet. The rainbow ® Super DCI-mini sensor can be used to spot-check all patients weighing 3 kg or more, further reducing the need for multiple sensor types; the sensor can be applied to an adult finger, a pediatric finger, or an infant finger, thumb, or great toe. The sensor is small and lightweight, with a flexible cable to provide sensor stability and patient comfort during monitoring. Next Generation SpHb technology offers motion tolerance and a faster time to display SpHb results (in as few as 30 seconds). In addition, field performance has been enhanced in lower hemoglobin ranges. Next Generation SpHb is enabled when the Rad-67™ Pulse CO-Oximeter ® and the DCI-mini or Super DCI-mini sensor are used together. SpCO monitoring may lead to the identification of elevated carbon monoxide levels that might otherwise go undetected in front-line settings, such as triage and emergency care. SpMet helps clinicians monitor for methemoglobin in care areas where the drugs that cause methemoglobinemia are most common, such as procedure labs and the operating room. Joe Kiani, Founder and CEO of Masimo, said, “This is an exciting day for us and hopefully a great opportunity to improve patient care. Since the invention of rainbow ® technology, we have been wanting our customers to be able to measure SpCO, SpMet, SpHb and SpO 2 simultaneously. Now they can! In addition, we have been pursuing a spot-check sensor that fits all patients across the age spectrum. We believe the rainbow ® Super DCI-mini sensor will be especially valuable for use in triage and emergency care situations. We plan to introduce a continuous measurement version of the Super Sensor in the near future.” The rainbow ® Super DCI-mini sensor, Next Generation SpHb, and Rad-67 have not received FDA 510(k) clearance and are not available for sale in the United States. @MasimoInnovates | #Masimo SpCO is intended to be used to monitor CO levels in the blood and is not intended to be used as the sole basis for making diagnosis or treatment decisions related to carbon monoxide poisoning. SpMet is not to be used as the sole basis for making diagnosis or treatment decisions related to methemoglobinemia. SpHb, SpCO, and SpMet monitoring are not intended to replace laboratory blood testing; blood samples should be analyzed by laboratory instruments prior to clinical decision making. About Masimo Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET ® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET ® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates, 1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs. 3,4,5 Masimo SET ® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world, 6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll. 7 In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb ®), oxygen content (SpOC™), carboxyhemoglobin (SpCO ®), methemoglobin (SpMet ®), and more recently, Pleth Variability Index (PVi ®) and Oxygen Reserve Index (ORi™), in addition to SpO 2, pulse rate, and perfusion index (PI). In 2014, Masimo introduced Root ®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO 2 ®pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/cpub/clinical-evidence.htm. ORi has not received FDA 510(k) clearance and is not available for sale in the United States. *The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium. References
Forward-Looking Statements This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo rainbow® Super DCI ®-mini sensor. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo rainbow ® Super DCI-mini sensor, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws. View source version on businesswire.com: http://www.businesswire.com/news/home/20170709005051/en/ Contacts Masimo |
|||||
