Aiosyn Expands its AI-Powered Quality Control Solution for Digital Pathology Slides to Support Immunohistochemistry (IHC) Staining
NIJMEGEN, Netherlands–(BUSINESS WIRE)– Aiosyn, a software company that develops AI-powered pathology solutions, has announced the latest advancement in their automated quality control (QC) algorithm. Aiosyn’s QC solution, AiosynQC, is now fully compatible with immunohistochemistry (IHC) slides, in addition to hematoxylin and eosin (H&E) slides. This update extends the benefits of AiosynQC and allows laboratories that rely significantly on IHC staining to further streamline operations.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230614656448/en/
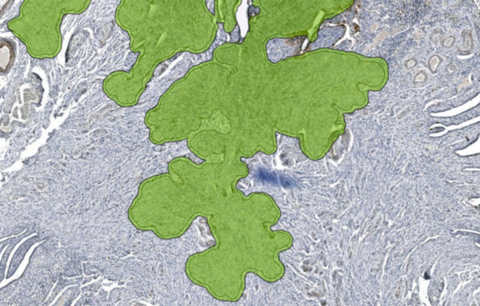
Unreadable regions affected by air bubbles on an IHC stained slide image are identified and highlighted with Aiosyn’s automated quality control solution powered by AI. (Graph: Business Wire)
“Immunohistochemistry is an integral part of modern pathology practices, and we are excited to offer an AI-powered solution for QC compatible with IHC staining, empowering laboratories to further improve their pathology workflow” says David Tellez, Chief Technology Officer of Aiosyn.
AiosynQC automatically recognizes and flags the most common artifacts in histology slide images, improving the quality and speed of the digital pathology workflow. The solution helps laboratories to ensure that only high-quality images are utilized by pathologists, technicians, and researchers. With the ability to flag problematic cases before they reach the pathologist, AiosynQC improves the efficiency of the workflow by reducing the manual check for artifacts during the quality control of slides.
IHC is the second most common staining technique in pathology laboratories after H&E and plays an important role in both routine diagnostic work as well as in basic and clinical research, including biomarker exploration. Therefore, the compatibility with such staining marks a significant milestone that highlights Aiosyn’s commitment to facilitate the adoption and integration of its software solutions into existing digital pathology workflows.
Alongside the expansion of support for IHC slides, Aiosyn is actively developing advanced deep learning algorithms for various pathologies. The recent launch of AiosynQC on Sectra Amplifier Marketplace further showcases Aiosyn’s dedication to making AI-powered technology accessible to pathology laboratories worldwide.
About Aiosyn
Aiosyn is a Dutch medical software company that develops AI-powered pathology solutions that will be integrated into standard pathology workflows. The Aiosyn team has been built upon 20+ years of research experience in the field of pathology and is rooted into the pathology practice.
About AiosynQC
AiosynQC is an AI-powered solution for automated quality control of digital histology slides. In the EU and the UK, AiosynQC is not considered a medical device under European IVDR and UK MDR 2002 legislation, respectively. AiosynQC is not intended to be used as an accessory to, nor is it necessary to be used in combination with any AI or other medical devices to specifically enable them to meet their intended purpose or directly assist in their functionality.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230614656448/en/
Contacts
Anna Correas, Marketing & Communications Specialist at Aiosyn
anna.correas@aiosyn.com

