Mainstay Medical kondigt publicatie aan van post-market klinische onderzoeksgegevens van lopende ReActiv8®-C-studie bij patiënten met chronische lage-rugpijn
Nieuw real-world bewijs ondersteunt verder de werkzaamheid en het gebruik van ReActiv8® Restorative Neurostimulation™ voor de behandeling van hardnekkige chronische lage-rugpijn
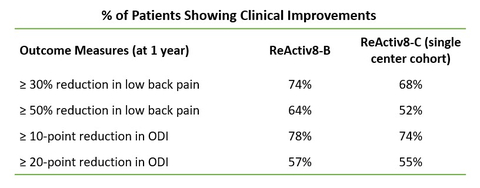
DUBLIN–(BUSINESS WIRE)– Mainstay Medical Holdings plc heeft vandaag de publicatie aangekondigd van gegevens van een real-world onderzoek in één centrum met klinische follow-up van één jaar van patiënten geselecteerd uit de ReActiv8®-C-studie. Patiënten bij wie ReActiv8 in Klinikum Itzehoe was geïmplanteerd, werden achtereenvolgens in dit cohort opgenomen als ze rugpijn ≥6 hadden en geen eerdere lumbale operatie hadden ondergaan. De eenjarige resultaten, gepubliceerd in World Neurosurgery, toonden aan dat een meerderheid van de 44 patiënten die werden gevolgd, statistisch significante verbeteringen vertoonden in pijn (NRS), invaliditeit (ODI) en kwaliteit van leven (EQ-5D-5L).
Dit persbericht bevat multimedia. Bekijk de volledige release hier: https://www.businesswire.com/news/home/20221122005230/en/
Mainstay Medical Announces Publication of Post-Market Clinical Trial Data from Ongoing ReActiv8®-C Study in Chronic Low Back Pain Patients
New real-world evidence further supports the efficacy and use of ReActiv8® Restorative Neurostimulation™ for the treatment of intractable Chronic Low Back Pain
DUBLIN–(BUSINESS WIRE)– Mainstay Medical Holdings plc today announced the publication of data from a single center, real-world study with one-year clinical follow-up of patients selected from the ReActiv8®-C study. Patients implanted with ReActiv8 at Klinikum Itzehoe were consecutively included into this cohort if they presented with back pain ≥6 and no prior lumbar surgery. The one-year results, published in World Neurosurgery, showed that a majority of the 44 patients followed up with demonstrated statistically significant improvements in pain (NRS), disability (ODI) and quality of life (EQ-5D-5L).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221122005230/en/
(Graphic: Business Wire)
This interim analysis indicates that the response to ReActiv8 for these patients is durable and the benefits improve over time, consistent with both a restorative mechanism of action and the ReActiv8-B randomized clinical trial results.
Dr. Med. Ardeshir Ardeshiri, Head physician of the Spine Surgery Section of the Clinic for Trauma Surgery and Orthopedics, Klinikum Itzehoe, said: “These favorable real-world data are consistent with the ReActiv8-B study, which is extremely important for me in adopting new technologies. I am excited to continue offering restorative neurostimulation with ReActiv8 for my patients with multifidus dysfunction resulting in chronic axial low back pain.”
Jason Hannon, CEO of Mainstay Medical, said: “These real-world results further validate ReActiv8’s restorative mechanism of action, which treats a primary underlying cause of mechanical chronic lower back pain, multifidus dysfunction. German physicians have been some of our foremost implanters of ReActiv8, and we look forward to continuing to make the therapy more broadly available to patients in this region.”
Link to publication of the study Real-World Evidence for Restorative Neurostimulation in Chronic Low Back Pain—a Consecutive Cohort Study – ScienceDirect
About ReActiv8®
ReActiv8 is an implantable medical device designed to treat adults with intractable chronic low back pain (CLBP) associated with multifidus muscle dysfunction. Multifidus muscle dysfunction may be evidenced by imaging or physiological testing in adults who have failed therapy including pain medications and physical therapy, and who are not candidates for spine surgery. ReActiv8 has received regulatory approval in several geographic areas, and is commercially available in the European Economic Area, Australia, the UK, and the US.
About Mainstay Medical
Mainstay Medical is a medical device company focused on commercializing its innovative implantable Restorative Neurostimulation™ system, ReActiv8®, for people with disabling mechanical CLBP. Mainstay Medical is headquartered in Dublin, Ireland and has subsidiaries operating in Ireland, the United States, Australia, Germany and the Netherlands.
Further information can be found at www.mainstaymedical.com.
Forward-Looking Statements
All statements in this announcement other than statements of historical fact are, or may be deemed to be, forward-looking statements. These forward-looking statements may include, without limitation, statements regarding the company’s intentions, beliefs or current expectations concerning, among other things, the company’s commercial efforts and performance, financial position, financing strategies, product design and development, regulatory applications and approvals, and reimbursement arrangements.
Forward-looking statements involve risk and uncertainty and are not guarantees of future performance. Actual results may differ materially from those described in, or suggested by, the forward-looking statements. A number of factors could cause results and developments to differ materially from those expressed or implied by the forward-looking statements herein, including, without limitation, the risks and uncertainties included in the company’s Annual Report for the year ended 31 December 2021, which should be read in conjunction with the company’s public disclosures (available on the company’s website (www.mainstaymedical.com). The forward-looking statements herein speak only as of the date of this announcement.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221122005230/en/
Contacts
PR and IR Enquiries:
LifeSci Advisors, LLC
Brian Ritchie
Tel: + 1 (212) 915-2578
Email: britchie@lifesciadvisors.com
FTI Consulting (for Ireland)
Jonathan Neilan or Patrick Berkery
Tel. : +353 1 765 0886
Email: mainstay@fticonsulting.com
Mainstay Medical
Corporate Communications
Email: Media@mainstaymedical.com

