Merz Aesthetics viert een nieuwe mijlpaal: wereldwijd worden tien miljoen spuiten van Radiesse® verzonden
FRANKFURT, Duitsland – (BUSINESS WIRE) – Merz Aesthetics, een wereldleider op het gebied van medische esthetiek, heeft vandaag aangekondigd dat Radiesse® viert dat er in bijna twintig jaar tijd tien miljoen injectiespuiten wereldwijd zijn verzonden. Radiesse® is een unieke injecteerbare vulstof met calciumhydroxylapatiet (CaHA ) die wordt gebruikt voor volumisering, lifting en contouren, evenals voor huidverjonging door natuurlijke collagenogenese te stimuleren. Het productportfolio omvat Radiesse® en Radiesse® Lidocaïne die effectieve en natuurlijk ogende cosmetische resultaten opleveren, die zich onderscheiden door het bewezen veiligheidsprofiel en de hoge patiënttevredenheid.
Dit persbericht bevat multimedia. Bekijk de volledige release hier: https://www.businesswire.com/news/home/20201020005619/en/
Merz Aesthetics Celebrates A New Milestone: Ten Million Syringes of Radiesse® Shipped Worldwide
FRANKFURT, Germany–(BUSINESS WIRE)– Merz Aesthetics, a global leader in medical aesthetics, today announced that Radiesse® is celebrating ten million syringes shipped worldwide over almost twenty years. Radiesse® is a unique calcium hydroxylapatite (CaHA) injectable filler1 used for volumization, lifting and contouring, as well as skin rejuvenation by stimulating natural collagenogenesis.2,3 The product portfolio includes Radiesse® and Radiesse® Lidocaine delivering effective and natural-looking cosmetic results, distinguished by its established safety profile4 and high patient satisfaction.5
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201020005619/en/
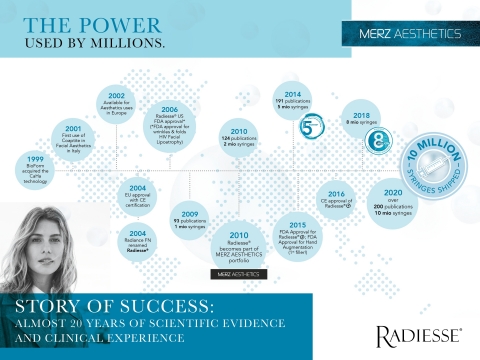
Radiesse History (Graphic: Business Wire)
“As we reflect on this milestone, we are proud of the fact that, Radiesse has proven to provide aesthetic practitioners with a unique treatment that they can administer confidently, while supporting their patients’ desired impact,” said Kristel Hectors, Head of Marketing EMEA, Merz Aesthetics. “With almost twenty years of scientific evidence and clinical experience, Radiesse is such a unique product and full of opportunities and possibilities. As we are looking ahead, we are excited for what’s to come over the next years for Radiesse.”
The milestone campaign launching under the tagline “The Power Used By Millions” reinforces the uniqueness of the product and its working mechanism. Informing health care professionals and educating patients on the uniqueness of Radiesse®, on its working mechanism and efficacy.
“Radiesse is my number one go to product because of its versatility. Radiesse plus for contouring, lifting, ligaments and volumizing. Classic Radiesse for classic indications like folds and of course hyperdiluted Radiesse for skin quality improvement,” said Jani van Loghem, MD Cosmetisch arts KNMG. “It is a biostimulatory product going beyond a hyaluronic acid: the collagen will be giving the patient the final result and it is a really nice message to the patient! That’s why I choose Radiesse.”
About Radiesse®/Radiesse® (+) Lidocaine
Radiesse® is an injectable dermal filler that provides immediate volume and correction and continues to work by stimulating the body’s own natural collagen production. In addition, Radiesse activated the self-renewing potential within the skin to make the skin firm, elastic and strong. Radiesse can be tailored to individual requirements and skin condition.1,6-10
Radiesse® (+) Lidocaine injectable implant is an opaque, dermal filler that contains a small quantity of local anesthetic (lidocaine). In Europe, Radiesse® (+) Lidocaine is indicated for plastic / reconstructive procedures, including deep dermal and subdermal soft tissue augmentation of the facial area and for restoration and correction of facial volume loss. The presence of lidocaine is intended to reduce patient pain and to enhance comfort during treatment.1,6-10
Radiesse® / Radiesse® (+) Lidocaine is valued by doctors and patients around the world for its tolerability and safety profile. Radiesse received the CE mark for aesthetic use in Europe in 2004, with U.S. FDA approval following in 2006. In that time, Radiesse has been considered a leading minimally-invasive aesthetic treatment trusted by healthcare providers around the world. The product has been available on the market for more than fifteen years, with now 10 million syringes shipped worldwide and a clinically proven safety profile supported by more than 200 clinical studies and peer-reviewed publications.1,6-10
Radiesse is available in more than 70 countries worldwide.
About Merz Aesthetics
At Merz Aesthetics, we are a medical aesthetics business with a long history of empowering health care professionals, patients and employees to live every day with confidence. We aim to help people around the world look, feel and live like the best versions of themselves. Clinically proven, our product portfolio includes injectables, devices and skin care treatments designed to meet each patient’s needs with the highest standards of safety and efficacy. We are known for building unique connections with customers who feel like family. Our global headquarters is in Raleigh, N.C., USA, with locations in 32 countries worldwide. Merz Aesthetics is part of Merz Group, a family owned company founded in 1908 and based in Frankfurt, Germany. Learn more at merzaesthetics.com.
Copyright © 2020 Merz Pharmaceuticals GmbH. All rights reserved. MERZ, MERZ AESTHETICS and the MERZ logo are registered trademarks of Merz Pharma GmbH & Co. KGaA. Radiesse® is a registered trademark of Merz North America, Inc.
1 RADIESSE Instructions for Use Nov. 2017. RADIESSE® Lidocaine Instructions for Use Jun.2018.
2 Berlin AL, et al. Dermatol Surg. 2008;34(suppl 1):S64-S67.
3 Moers-Carpi M, et al. Dermatol Surg. 2007;33(suppl 2):S144-S151.
4 Van Loghem JV, et al. J Clin Aesthet Dermatol. 2015:8(1)38-49.
5 Juhász MLW, Marmur ES, Dermatol Surg. 2018;44(8)1084-93.
6 Yutskovskaya Y, et al. J Drugs Dermatol. 2014;13(9):1047-52.
7 Yutskovskaya YA, Kogan EA. J Drugs Dermatol. 2017;16(1):68-74.
8 Schachter D, et al. J Drugs Dermatol. 2016 Aug 1; 15(8): 1005-10.
9 Sundaram H, et al. Dermatol Surg. 2010;36 (suppl 3):1859-1865.
10 Schachter Science Brief: Pain Control using the Injectable Dermal Filler Radiesse (+) for the correction of Nasolabial folds. Daniel Schachter, Vince Bertucci, Nowell Solish. POSTER PRESENTATION at 13th Anti-Aging Medicine World Congress (2015), Monaco, Monte Carlo.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201020005619/en/
Contacts
Media Contact
Merz Pharmaceuticals GmbH
Regional Communications EMEA
Felicia Glatzel
Email: felicia.glatzel@merz.de

