RevBio krijgt goedkeuring van de regelgevende instantie van het VK om een klinische proef te starten voor een geoptimaliseerde formulering van zijn biomateriaal voor tandbeenlijm
RevBio krijgt goedkeuring om een klinische proef in het Verenigd Koninkrijk te starten om een aangepaste formulering van zijn botadhesief biomateriaal voor implantaattandheelkunde te bestuderen
LOWELL, Massachusetts–(BUSINESS WIRE)– RevBio, Inc., heeft aangekondigd dat het goedkeuring heeft gekregen van de Medicines and Healthcare products Regulatory Agency in het Verenigd Koninkrijk om een klinische proef met 15 patiënten te starten om de veiligheid en werkzaamheid van onmiddellijk gestabiliseerde tandheelkundige implantaten na tandextracties met behulp van een geoptimaliseerde formulering van Tetraniet®, het biomateriaal van het bedrijf dat hecht aan bot.
Dr. Michael R. Norton, BDS, FDS, RCS(Ed), een in Londen gevestigde kaakchirurg, bekende docent en voormalig voorzitter van de Academy of Osseointegration, zal als hoofdonderzoeker voor deze klinische proef dienen. “Ik vind het geweldig om betrokken te zijn bij deze klinische proef, die voortbouwt op de eerste die ik eerder dit jaar heb afgerond,” zei Dr. Norton. “Sinds de start van de eerdere door MHRA goedgekeurde studie in het Verenigd Koninkrijk, heeft RevBio het osteopromotorische potentieel van zijn biomateriaal aanzienlijk verbeterd.”
RevBio Receives Approval from the U.K.’s Regulatory Authority to Initiate a Clinical Trial for an Optimized Formulation of its Dental Bone Adhesive Biomaterial
RevBio Receives Approval to Start a Clinical Trial in the United Kingdom to Study a Modified Formulation of its Bone Adhesive Biomaterial for Implant Dentistry
LOWELL, Mass.–(BUSINESS WIRE)– RevBio, Inc., announced that it has received approval from the Medicines and Healthcare products Regulatory Agency in the United Kingdom to start a 15-patient clinical trial to examine the safety and efficacy of immediately stabilized dental implants following tooth extractions using an optimized formulation of Tetranite®, the company’s bone adhesive biomaterial.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220915005733/en/
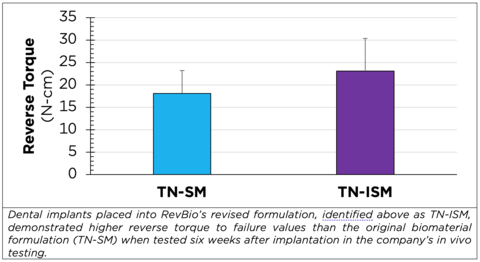
(Graphic: Business Wire)
Dr. Michael R. Norton, BDS, FDS, RCS(Ed), a London-based oral surgeon, noted lecturer, and former President of the Academy of Osseointegration, will serve as the chief investigator for this clinical trial. “I am thrilled to be involved in this clinical trial which builds on the first one I completed earlier this year,” said Dr. Norton. “Since initiating the prior MHRA-approved study in the United Kingdom, RevBio has significantly improved the osteopromotive potential of its biomaterial.”
RevBio has optimized its bone adhesive biomaterial primarily by adjusting the pH of the material as it undergoes its self-setting reaction. In addition, the company has also increased the porosity of the material. Benchtop and animal testing has shown that these changes have improved the material’s biocompatibility with the gingival tissues that surround tooth extraction sites. These formulation changes have also increased the substitution rate of new bone formation without sacrificing the biomaterial’s ability to adhere to bone. In fact, this formulation is more adhesive than the company’s prior dental formulation.
Similar to Dr. Norton’s prior study, this new clinical trial will focus specifically on anterior teeth located in the aesthetic or “smile” zone where the loss of a tooth is highly visible. Patients will receive temporary crowns at the time their implants are placed which will obviate a costly, complex, and lengthy bone grafting process for many patients, greatly accelerating the overall treatment timeframe.
“We are excited to partner again with Dr. Norton who is a thought leader in advancing clinical practice in the field of implant dentistry,” said Alan Pollack, RevBio’s Senior Director of Dental Clinical Operations. “The development of this new formulation has really advanced the company’s ability to tune the biomaterial’s performance to address anatomically-specific requirements.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called Tetranite®. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio’s Tetranite technology is not yet approved for commercial use.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220915005733/en/
Contacts
Michael Tiedemann
6177630923

