Vroege haalbaarheidsstudie toont succesvol gebruik van de preCARDIA-technologie van Abiomed aan
DANVERS, Massachusetts–(BUSINESS WIRE)– Abiomed (NASDAQ: ABMD) kondigt de succesvolle resultaten aan van de eerste menselijke vroege haalbaarheidsstudie van het preCARDIA-systeem. Het preCARDIA-systeem is ontworpen om decongestie te verbeteren bij patiënten met acutely decompensated heart failure (ADHF) door met tussenpozen de superieure vena cava af te sluiten. De resultaten van het onderzoek zijn deze week gepubliceerd in het tijdschrift Circulation: Heart Failure.
Dit persbericht bevat multimedia. Bekijk de volledige release hier: https://www.businesswire.com/news/home/20220112005319/en/
Ondanks de beschikbare farmaceutische behandelingen, is hartfalen de belangrijkste oorzaak van ziekenhuisopname bij patiënten ouder dan 65 jaar. Diuretica kunnen de symptomen van hartfalen en de hartfunctie helpen verbeteren door vochtophoping te verminderen. Diuretica hebben echter tijd nodig om te werken en kunnen niet effectief zijn bij patiënten met chronisch hartfalen. preCARDIA is ontworpen om de vuldruk te verlagen wanneer bloed het hart en de longen binnenkomt, waardoor het hart en de nieren mogelijk effectiever kunnen werken, en mogelijk therapie biedt voor patiënten die niet reageren op diuretica, naar schatting ongeveer 300.000 van de één miljoen ADHF-opnames in de VS per jaar.
Early Feasibility Study Demonstrates Successful Use of Abiomed’s preCARDIA Technology
DANVERS, Mass.–(BUSINESS WIRE)– Abiomed (NASDAQ: ABMD) announces the successful results of the first-in-human early feasibility study of the preCARDIA system. The preCARDIA system is designed to improve decongestion in acutely decompensated heart failure (ADHF) patients by intermittently occluding the superior vena cava. The results of the study published this week in the journal Circulation: Heart Failure.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220112005319/en/
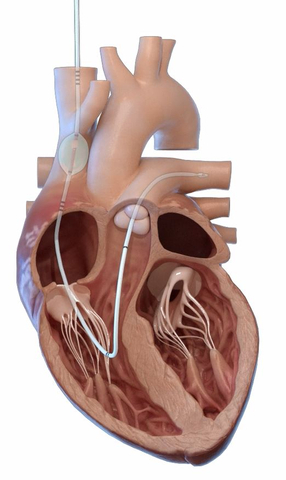
The preCARDIA system is placed in the heart to intermittently occlude the superior vena cava. (Graphic: Business Wire)
Despite available pharmaceutical treatments, heart failure is the leading cause of hospitalization in patients older than 65 years of age. Diuretics may help to improve heart failure symptoms and heart function by reducing fluid overload. However, diuretics take time to work and may be ineffective in patients with chronic heart failure. preCARDIA is designed to reduce filling pressure as blood enters the heart and lungs, which may enable the heart and kidneys to work more effectively, potentially providing therapy for patients non-responsive to diuretics, estimated to be approximately 300,000 of the one million U.S. ADHF admissions per year.
The multicenter, prospective, single-arm VENUS-HF Early Feasibility Study examined 30 patients with ADHF who were assigned preCARDIA therapy for 12 or 24 hours. The primary endpoint was a composite of major adverse events through 30 days. The study found:
- Freedom from device- or procedure-related major adverse events in 100% of patients (n=30/30)
- Successful placement, activation and removal of preCARDIA in 97% of patients (n=29/30)
- Right atrial pressure decreased 34% from baseline (p<0.001)
- Pulmonary capillary wedge pressure decreased 27% from baseline (p<0.001)
- Urine output increased by 130% from pretreatment values (p<0.01)
- Net fluid output increased by 156% from pretreatment values (p<0.01)
“The study met the safety and feasibility endpoints and suggests for the first time that with the preCARDIA system it is possible to rapidly reduce cardiac filling pressures and augment urine output by intermittently occluding the superior vena cava in patients with ADHF,” said Navin Kapur, MD, the study’s lead author and executive director of the CardioVascular Center for Research and Innovation at Tufts Medical Center.
The results support additional study of preCARDIA. In November, the United States Food and Drug Administration (FDA) authorized preCARDIA’s early feasibility study to be expanded by 30 additional patients to a total of 60 patients.
preCARDIA is an investigational device, limited by federal law to investigational use only.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc., is a leading provider of medical devices that provide circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220112005319/en/
Contacts
Media:
Tom Langford
Director of Communications
+1 (978) 882-8408
Investors:
Todd Trapp
Vice President and Chief Financial Officer
+1 (978) 646-1680

