Grote, multicenter- en multi vereniging studie van patiënten met impella-ondersteuning toont 30-daagse AMI-overleving bij cardiogene schokken van 81%
BOSTON–(BUSINESS WIRE)– Resultaten van een drie jaar durend, door een onderzoeker geleid onderzoek van alle door Impella ondersteunde patiënten die werden behandeld in 109 ziekenhuizen in Japan (n=1344), laten een overlevingspercentage van 30 dagen zien van 81% voor AMI cardiogene shock (AMICS ) patiënten. De studie is een update van een tussentijdse analyse uit 2020 en werd gepresenteerd op de Transcatheter Cardiovascular Therapeutics (TCT)-conferentie in 2022 in Boston.
Dit persbericht bevat multimedia. Bekijk de volledige release hier: https://www.businesswire.com/news/home/20220919005279/en/
De analyse onderzocht 293 opeenvolgende door Impella ondersteunde AMICS-patiënten in het J-PVAD-register, een register uitgevoerd door 10 Japanse professionele verenigingen, waaronder de Japanese Circulation Society (JCS). Resultaten toonden 81% overleving na 30 dagen. Historische overlevingspercentages voor cardiogene shock zonder Impella zijn ongeveer 50%.
Large, Multi-Center, Multi-Society Study of Impella-supported Patients Finds 30-day AMI Cardiogenic Shock Survival of 81%
BOSTON–(BUSINESS WIRE)– Results of a three-year, investigator-led study of all Impella-supported patients treated at 109 hospitals in Japan (n=1,344) show 30-day survival rates of 81% for AMI cardiogenic shock (AMICS) patients. The study is an update to a 2020 interim analysis and was presented at the 2022 Transcatheter Cardiovascular Therapeutics (TCT) conference taking place in Boston.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220919005279/en/
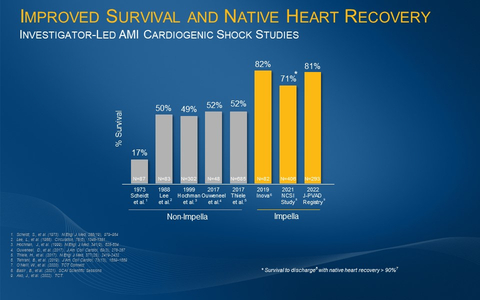
Caption: The investigator-led Inova, NCSI and J-PVAD studies all demonstrate an improvement from the historical AMI cardiogenic shock survival rate of approximately 50% when patients are treated with best practices including Impella. (Graphic: Business Wire)
The analysis examined 293 consecutive Impella-supported AMICS patients in the J-PVAD Registry, a registry conducted by 10 Japanese professional societies, including the Japanese Circulation Society (JCS). Results demonstrated 81% survival at 30 days. Historical cardiogenic shock survival rates without Impella are approximately 50%.
“The results of this study demonstrate that when Impella is used and best practices are followed, it is possible to achieve heart recovery and greater than 80% survival rates for patients with AMI cardiogenic shock,” said lead investigator Junya Ako, MD, an interventional cardiologist and chair of the department of cardiovascular medicine at Kitasato University Hospital in Kanagawa.
These results are consistent with other published investigator-led studies, such as the National Cardiogenic Shock Initiative Study (NCSI) and the Inova study by Tehrani et al., that have demonstrated significant increases in survival with the use of Impella and best practices such as placing Impella prior to percutaneous coronary intervention (PCI) (see figure 1).
J-PVAD data is independently monitored and shared with the Japan Pharmaceuticals and Medical Devices Agency (PMDA).
ABOUT IMPELLA HEART PUMPS
Impella 2.5, Impella CP®, Impella CP with SmartAssist, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are U.S. FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed (ABMD), is a leading provider of medical technology that provides circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: http://www.abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220919005279/en/
Contacts
For further information:
Media:
Jenny Leary
Associate Director, U.S. Communications
+1 (978) 882-8491
Investor:
Todd Trapp
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680

