CardioMEMS HF System van St. Jude Medical in Europese richtlijn voor hartpatiënten
ST. PAUL, Minn.–(BUSINESS WIRE)– St. Jude Medical, Inc., een internationaal bedrijf in medische apparatuur, heeft vandaag bekendgemaakt dat zijn CardioMEMS ™ HF System is opgenomen in de richtlijn van de European Society of Cardiology (ESC) als een gericht middel voor therapie en controle van hartpatiënten. De nieuwe richtlijn voor diagnose en behandeling van acuut en chronisch hartfalen bevat voor het eerst het CardioMEMS HF System voor controle van de druk in het longslagader.
Voor deze klasse IIb-aanbeveling van CardioMEMS HF binnen de in de richtlijn aangewezen therapie voor hartpatiënten zijn robuuste data van het CHAMPION-onderzoek gebruikt.
St. Jude Medical CardioMEMS HF System is Now Guideline Directed Therapy for Heart Failure Patients in Europe
ST. PAUL, Minn.–(BUSINESS WIRE)– St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, today announced that its CardioMEMS ™ HF System was added to the European Society of Cardiology (ESC) guidelines as a directed therapy management and monitoring tool for heart failure patients. The new 2016 ESC Clinical Practice Guidelines for the diagnosis and treatment of acute and chronic heart failure for the first time include pulmonary artery pressure monitoring with the CardioMEMS HF System.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20160606005438/en/
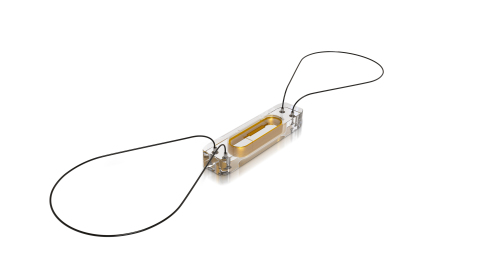 The CardioMEMS HF System uses a miniaturized, wireless monitoring sensor that is implanted in the pulmonary artery. (Photo: Business Wire)
The CardioMEMS HF System uses a miniaturized, wireless monitoring sensor that is implanted in the pulmonary artery. (Photo: Business Wire)Robust clinical data from the CHAMPION study was used to establish this Class IIb recommendation for the CardioMEMS HF System within the guideline directed therapy for heart failure patients.
“The ESC guideline for the CardioMEMS HF System provides direction for physicians who are working to appropriately treat our heart failure patients and reduce their risk of repeated heart failure hospitalizations,” said Dr. Giovanni Battista Perego from Istituto Auxologico Italiano, Milan, Italy.
The data supporting this decision include evidence based on the CHAMPION study and applies to all Class III heart failure patients regardless of their ejection fraction (measurement of how much blood is pumped out of the heart).
CHAMPION clinical data publications include:
- Wireless pulmonary artery hemodynamic monitoring chronic heart failure: a randomized controlled trial (The Lancet)
- Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomized trial (Journal of the American College of Cardiology)
“We are very pleased to see the CardioMEMS HF System was included in the guidelines and congratulate the European Society of Cardiology for working to make this technology more widely available to benefit patients living with heart failure,” said Dr. Philip B. Adamson, medical director and vice president of medical affairs for St. Jude Medical.
The CardioMEMS HF System is the first and only CE Mark approved heart failure monitor that, when used by physicians, has been shown to significantly reduce heart failure hospital admissions and improve the quality of life in New York Heart Association (NYHA) Class III patients. Long-term, prospective data published in The Lancet supports the effectiveness of the CardioMEMS HF System at reducing heart failure hospitalizations by demonstrating the system can provide physicians with the hemodynamic data to proactively manage their heart failure patients.
The CHAMPION trial originally demonstrated a statistically and clinically significant 28 percent reduction in the rate of HF hospitalizations at six months, and a 37 percent reduction in HF hospitalizations during an average follow-up duration of 15 months. The CardioMEMS HF System is the only proven treatment for New York Heart Association (NYHA) Class III HeartFailure patients who have a preserved Ejection Fraction (HFpEF), which makes up approximately half of all heart failure patients.
In addition, recently published data in Circulation: Heart Failure showed that management with the CardioMEMS HF System significantly reduced 30-day hospital readmission rates for Medicare-eligible patients.
Approximately 23 million people worldwide are afflicted with congestive heart failure and 2 million new cases are diagnosed worldwide each year.
About St. Jude Medical’s Heart Failure Business
St. Jude Medical is pioneering heart failure disease management with innovative solutions like the CardioMEMS HF System, ground-breaking quadripolar technology and, in select European markets, the HeartMate 3 ™ left ventricular assist system and our first-to-market MultiPoint ™ pacing technology. St. Jude Medical collaborates with heart failure specialists, clinicians and advocacy partners to provide innovative, cost-effective solutions that help reduce hospitalizations and improve patient quality of life for heart failure patients around the world.
For more information about St. Jude Medical’s focus on heart failure, visit the St. Jude Medical Heart Failure Media Kit or the St. Jude Medical PULSE Blog.
Information for patients to learn more about heart failure can be found at www.heartfailureanswers.com.
About St. Jude Medical
St. Jude Medical is a leading global medical device manufacturer and is dedicated to transforming the treatment of some of the world’s most expensive epidemic diseases. The company does this by developing cost-effective medical technologies that save and improve lives of patients around the world. Headquartered in St. Paul, Minn., St. Jude Medical employs approximately 18,000 people worldwide and has five major areas of focus that include heart failure, atrial fibrillation, neuromodulation, traditional cardiac rhythm management and cardiovascular. For more information, please visit sjm.com or follow us on Twitter @SJM_Media.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, anticipated regulatory approvals and future product launches, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management’s current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include market conditions and other factors beyond the Company’s control and the risk factors and other cautionary statements described in the Company’s filings with the SEC, including those described in the Risk Factors and Cautionary Statements sections of the Company’s Annual Report on Form 10-K for the fiscal year ended January 2, 2016 and Quarterly Report on Form 10-Q for the fiscal quarter ended April 2, 2016. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.
View source version on businesswire.com: http://www.businesswire.com/news/home/20160606005438/en/
Contacts
St. Jude Medical, Inc.
J.C. Weigelt, 651-756-4347
Investor Relations
jweigelt@sjm.com
or
Kristi Warner, 651-756-2085
Media Relations
kwarner@sjm.com
