Women’s Health Tech Startup Womed toont klinische onderzoeksresultaten van eerste vruchtbaarheidsbehoud, intra-uterien technologieproduct
Womed Leaf™ is veilig en effectief gebleken in het voorkomen van intra-uteriene verklevingen, krijgt CE-markering
MONTPELLIER, Frankrijk–(BUSINESS WIRE)– Womed, het gezondheidstechnologiebedrijf voor vrouwen dat baanbrekende innovatieve behandelingen tegen uitdagende baarmoederpathologieën op basis van een nieuwe technologie voor polymeermateriaal pioniert, heeft vandaag de publicatie aangekondigd in het Journal of Minimally Invasive Gynecology (JMIG) van de resultaten van de klinische proef ter evaluatie van het eerste product, Womed Leaf™. De klinische gegevens toonden aan dat het apparaat veilig en effectief is in het voorkomen van intra-uteriene adhesie bij vrouwen met baarmoederfibromen die hysteroscopische myomectomie ondergaan. Womed kondigde ook aan dat Womed Leaf™ de CE-markering heeft gekregen.
Intra-uteriene verklevingen (IUA’s) treden op na 20% tot 45% van de chirurgische ingrepen, wanneer de baarmoederwanden aan elkaar binden als gevolg van abnormale littekens. IUA’s zijn een belangrijke oorzaak van onvruchtbaarheid en herhaalde miskramen. IUA-behandeling, met een recidiefpercentage van wel 60%, laat vrouwen onzeker en angstig over hun kans om zwanger te worden.
Women’s Health Tech Startup Womed Showcases Clinical Trial Results of First Fertility Preserving, Intrauterine Technology Product
Womed Leaf™ is shown to be safe and effective in preventing intrauterine adhesions, receives CE Mark clearance
MONTPELLIER, France–(BUSINESS WIRE)– Womed, the women’s health tech company pioneering innovative treatments against challenging uterine pathologies based on a novel polymer material technology, today announced the publication in the Journal of Minimally Invasive Gynecology (JMIG) of results of the clinical trial evaluating its first product, Womed Leaf™. The clinical data demonstrated that the device is safe and effective in preventing intrauterine adhesion in women with uterine fibroids undergoing hysteroscopic myomectomy. Womed also announced that Womed Leaf™ received CE Marking.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210907005710/en/
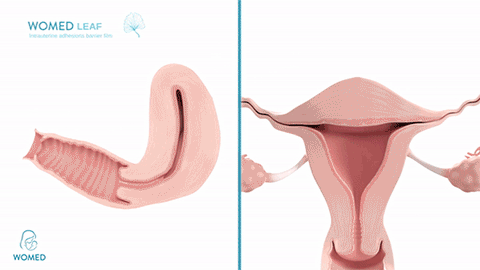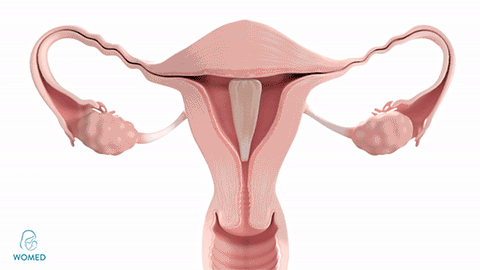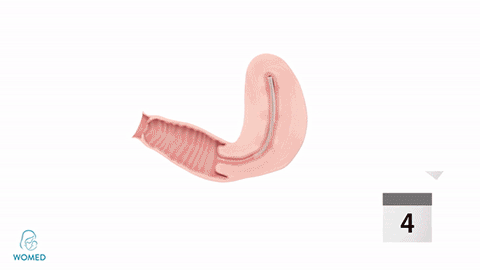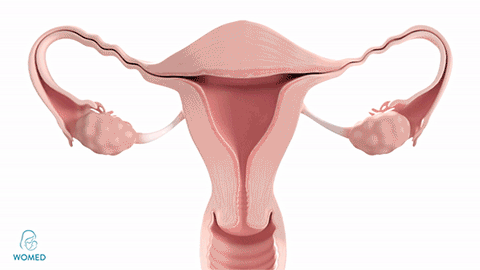
Womed Leaf mode of action
Intrauterine adhesions (IUAs) occur after 20% to 45% of surgical interventions, when the uterine walls bind together due to abnormal scarring. IUAs are a major cause of infertility and repeated miscarriages. IUA treatment, with a recurrence rate as high as 60%, leaves women unsure and anxious about their chance to conceive.
To prevent IUA formation, ObGyns need to keep the uterine walls separated after an intrauterine intervention. They can use various kinds of gels, but these existing materials become liquid and typically flow out of the cavity too quickly to create an effective barrier. Womed Leaf™ is the first and only mechanical barrier against IUA as it is composed of a soft film that protects the entire uterine cavity for a week and is painlessly discharged naturally afterwards.
Womed LeafTM was evaluated during the first trial of Womed’s clinical program, PREG1, which enrolled 23 patients who were scheduled for hysteroscopic myomectomy. Device manipulation was less than two minutes in all cases, and the procedure was described as easy by all operators. None of the study participants suffered any injuries, reactions or adverse events attributable to Womed Leaf. The device caused zero to minimal discomfort to patients and effectively prevented adhesion formation in 83% of women at six weeks.
“We have been impressed with Womed Leaf’s ease of use and are now very confident in its safety profile,” said Prof. Herve Fernandez, head of the Obstetrics and Gynecology Department at the Bicetre University Hospital, AP-HP, Paris, France. “A mechanical barrier that protects the cavity during the entire healing phase is very compelling compared to our current options.”
“Womed Leaf™ represents a major breakthrough in helping women preserve or recover their fertility from the heartbreaking condition of IUA, and we are thrilled by its clinical results and CE Mark approval,” said Gonzague Issenmann, co-founder and Executive Chairman of Womed. “We now feel confident to carry out our ambitious strategy: to leverage Womed’s proprietary polymer technology and develop a portfolio of unique intrauterine drug delivery products to safely treat challenging uterine pathologies, like acute bleeding and endometriosis.”
About WOMED
Womed was founded by health tech entrepreneur Gonzague Issenmann, biomedical polymer specialist Prof. Xavier Garric and reproductive specialist Dr. Stephanie Huberlant, to commercialize an invention from Garric’s Polymers for Health and Biomaterials lab at the University of Montpellier, France. Womed’s proprietary degradable polymer technology is designed specifically for temporary uterine implantation and controlled drug delivery.
The first product, Womed Leaf™, is a medical device to treat and prevent bonding of the uterine walls after surgical intervention, which is responsible for one in five miscarriages. Womed’s pipeline of intrauterine drug delivery products include treatments for acute uterine bleeding and endometriosis.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210907005710/en/
Contacts
Press contact:
Gonzague Issenmann, news@womedtech.com
Check out our twitter: @NewsNovumpr
