Bioventus investeert in nieuw klinisch-wetenschappelijk onderzoen naar EXOGEN®
DURHAM, N.C.–(BUSINESS WIRE)– Bioventus, wereldwijd toonaangevend in orthobiologische producten, heeft vandaag aangekondigd opdracht te geven tot een innovatieve reeks praktisch bewezen, direct bij patiënten uitgevoerde onderzoeken die onderschrijven dat het EXOGEN Ultrasound Bone Healing System het risico op pseudartrose na een botbreuk verkleint bij aanwezigheid van andere bekende risicofactoren. EXOGEN, dat in 1994 is ‘pre-market approval’ kreeg van de FDA, is al meer dan twintig jaar ingezet bij de behandeling van meer dan 1 miljoen patiënten over de hele wereld. Het product maakt gebruikt van veilige, effectieve gepulseerde geluidsgolven van lage sterkte voor de stimulering van de natuurlijke genezingsprocessen in het lichaam.
Bioventus to Invest in New Clinical Research for EXOGEN® |
|||||
|
DURHAM, N.C.–(BUSINESS WIRE)– Bioventus, a global leader in orthobiologics, today announced it will commission an innovative series of real-world evidence, direct-to-patient studies to further validate the ability of its EXOGEN Ultrasound Bone Healing System to mitigate the risk of a fracture progressing to nonunion in the presence of known risk factors. FDA PMA approved in 1994, EXOGEN has provided treatment to more than 1 million patients worldwide for more than 20 years and has a long clinical history. The product uses safe, effective low-intensity pulsed ultrasound (LIPUS) to help stimulate the body’s natural healing process. 1 This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170315005783/en/ 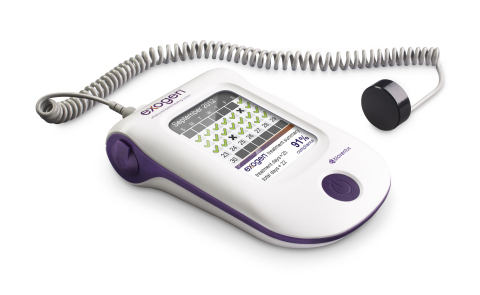 EXOGEN Ultrasound Bone Healing System from Bioventus (Photo: Business Wire) EXOGEN Ultrasound Bone Healing System from Bioventus (Photo: Business Wire)
Recent publications analyzing data from a large registry of almost 8,000 fractures treated withEXOGEN suggest that the device supports healing fractures in patients despite the presence of associated comorbidities or medication use. 2,3,4 This new clinical research, known as the Bioventus Observational Non-interventional EXOGEN Studies (BONES), will build on this evidence and supplement the product’s broad body of clinical knowledge in a prospective population-based innovative clinical development program. The BONES studies will compare the incidence of fracture nonunions in patients utilizing the EXOGEN device with patients from a national health insurance claims database who received standard of care alone. The studies will include bones representative of long and small bones of upper and lower extremities. The unique design was discussed with FDA during its development. “BONES represents a significant investment in developing epidemiologically grounded rigorous clinical evidence to support use of EXOGEN in fractures at risk, and to underscore the product’s clinical utility in mitigating the risk of nonunions, a highly debilitating and costly condition,” said Alessandra Pavesio, Senior Vice President and Chief Science Officer, Bioventus. “It will build upon knowledge gained from extensive research conducted by Bioventus and recently published in JAMA Surgery, in over 700,000 fracture patients that has identified 40-plus factors which place a patient at an increased risk of progression to a nonunion. 5” About Bioventus Bioventus is an orthobiologics company that delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. Bioventus has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN ® Ultrasound Bone Healing System is the #1 prescribed bone healing system in the US and is the only FDA-approved bone healing device that uses safe, effective ultrasound to stimulate the body’s natural healing process. Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.BioventusGlobal.com and follow the company on Twitter @Bioventusglobal. Bioventus, the Bioventus logo, and EXOGEN are registered trademarks of Bioventus LLC.
EXOGEN – Summary of Indications for Use in the US *Summary of Indications for Use: The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established non-unions* excluding skull and vertebra. In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopaedically managed by closed reduction and cast immobilization. There are no known contraindications for the EXOGEN device. Safety and effectiveness has not been established for individuals lacking skeletal maturity; pregnant or nursing women; patients with cardiac pacemakers; on fractures due to bone cancer; or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel. Full prescribing information can be found in product labeling, at www.exogen.com or by contacting customer service at 1-800-836-4080. *A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing. View source version on businesswire.com: http://www.businesswire.com/news/home/20170315005783/en/ Contacts Bioventus |
|||||
